Glycolysis/Fermentation with Molecular Models
“Glycolysis” strikes fear into many undergrad biology students because it presents them with an abstract series of reactions and molecules which are difficult to visualize and therefore incorporate into a coherant biochemical framework. This exercise has each student taking responsibility for a single molecule in the series, learning the following about it:
1) its structure
2) its precursor
3) the enzyme which created it
4) the enzyme which will act on it
5) the product of that action
6) the significance of the bond structure, particularly those involving phosphate. (Note whether phosphoester (low energy) or phosphoanhydride (high energy).)
They are then to describe these features to their fellow students in sequence. This strategy for teaching glycolysis has received many positive reviews from students who have used it.
See the bottom of the page for a key to the elements and directions for the construction of the models.
Here is the sequential listing of the molecules of glycolysis

1) Glucose
Glucose is both an aldose and a hexose. It enters the cell by diffusion, and the action of hexokinase holds it there.
Hexokinase transfers a phosphate from ATP to the number six carbon on glucose. This not only initiates glycolysis, but traps glucose in the cell since the ionic phosphate group makes diffusion out of the cell impossible without assistance.

2) Glucose-6-phosphate (G-6-P)
Phosphoglucoisomerase moves the carbonyl from the number one to the number two carbon, changing the molecule from an aldose to a ketose. This will free up the number one carbon for the phosphoryllation of the next step.

3) Fructose-6-phosphate (F-6-P)
Phosphofructokinase-1 is a critical enzyme in several ways. It transfers a phosphate from ATP to the number one carbon, thus placing ionic “handles” on either end (a PO4 on both the number 1 and number 6 carbons), allowing for the “breaking” of the molecule in the next step. This enzyme is allosterically inhibited by elevated ATP concentration.

4) Fructose-1,6-bisphosphate (F1,6bisP)
Aldolase splits fructose-1,6-bisphosphate into two pieces, dihydroxyacetonephosphate (DHAP, a ketone) and glyceraldehyde-3-phosphate of the next step.


5) Dihydroxyacetone phosphate (DHAP)
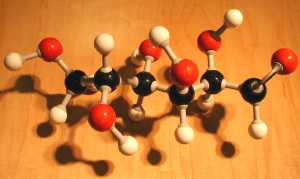
The image at the upper left shows fructose 1,6-phosphate at the bottom, splitting into dihydroxyacetone phosphate above on the left, and glyceraldehye-3-phosphate above on the right.
Triose phosphate isomerase moves the carbonyl from the number two carbon to the number one carbon, isomerizing DHAP to glyceraldehyde-3-phosphate. Thus, a single glucose generates two glyceraldehude-3-phosphates and all the following reactions are doubled.
The lower image shows dihydroxyacetone phosphate above on the left, and glyceraldehye-3-phosphate close up.

6) Glyceraldehyde-3-phosphate (3-GAP)
Glyceraldehyde-3-phosphate dehydrogenase performs a complex reaction in which glyceraldehyde-3-phosphate is oxidized by the removal of the hydrogen from the aldehyde. This hydrogen is used to reduce NAD+. A phosphate is added to the number one carbon in place of the hydrogen. This produces a very high energy phosphoacid anhydride.

7) 1,3-bisphosphoglycerate (1,3bisPGA)
Phosphoglycerokinase transfers the high energy phosphate from the phosphoanhydride bond on the number one carbon to an ADP (substrate level phosphoryllation).
Note that this is the first ATP to be generated, and two are created for every glucose molecule which entered the pathway.

8) 3-phosphoglycerate (3-PGA)
Phosphoglyceromutase moves the phosphate from the number three carbon to the number two carbon to prepare it for dehydration in the step after next.

9) 2-phosphoglycerate (2-PGA)
Enolase dehydrates 2-phosphoglycerate to form phosphoenolpyruvate.

10) Phosphoenolpyruvate (PEP)
Pyruvate kinase transfers the high energy phosphate from PEP to ADP, yielding pyruvate and ATP.
Phosphoenolpyruvate is the most energetic molecule in all of the molecules in glucose catabolism, because of the strain in the enol and the phosphate being adjacent to an ethylene bond.
It can engage in substrate level phosphoryllation, donating its phosphate to an ADP yielding ATP.

11) Pyruvate
Pyruvate is the end product of glycolysis, and, in the presense of oxygen, will be dehydrogenated by pyruvate dehydrogenase to yield acetyl coenzyme A, the “crossroads” molecule of carbon metabolism.

12) Lactic acid
Lactate dehydrogenase regenerates NAD+ (required for oxidation of glyceraldehyde-3-phosphate by (named for the opposite reaction) reducing pyruvate. This happens in muscle during intense exercise (ergo, muscle burn) and in milk during fermentation (butermilk and yogurt).

13) Acetaldehyde
Pyruvate decarboxylase decarboxylates pyruvate in yeast to produce acetaldehyde. Thiamine is required for decarboxylation, and yeast synthesizes it in large quantities, making nutritional yeast an excellent source for this vitamin.

14) Carbon dioxide & Ethanol
Alcohol dehydrogenase oxidizes acetaldehyde and concomitantly oxidizes NADH to yield ethyl alcohol and NAD+, required for oxidation of glyceraldehude-3-phosphate thus allowing glycolysis to continue in the absense of oxygen or other hydrogen acceptor.
Once students have constructed the assigned molecule, and learned the enzymes and related molecules, the models are laid out in sequence on a big table, and students in succession gave the following information:
1) The name of the molecule they had constructed
2) Its characteristic features
3) How it differs from the previous molecule
5) The enzyme which produced it
6) How it will be changed into the next molecule and why
7) The name of the enzyme which performs this change and the meaning of the name of the enzyme.
Students were to discuss selected phosphorylated molecules (i.e. glucose 6 phosphate, 1,3 bisphosphoglycerate, and phosphoenolpyruvate), naming and discussing the bonds by which phosphate is attached, and the relative energy content in each type (phosphoester, phosphoanhydride, resons for PEP’s unusual energy). The products of hydrolysis of these bonds was demonstrated and discussed.
Following the discussion, students leave the room, the molecules are randomized, and students return and identify the molecules as a way of demonstrating what they have learned.
KEY TO CONSTRUCTION OF MODEL MOLECULES:
Here is the key for the identities of the elements in the models
For the construction, remember that in straight chain illustrations of the chemical structures:
The carbon back bone is vertical with vertical bonds from each carbon projecting away from the observer.
The horizontal bonds project towards the observer.
Review the lab protocol on the Krebs cycle molecules.
Videos on glycolysis from Films for the Humanities and Sciences series which review the process are:
VIDT QH 633 .C45 1992 pt.1 Cell and Energy (Series Title-Cellular Respiration)
VIDT QH 633 .C45 1992 pt.2 Glycolosis 1 (Series Title-Cellular Respiration)
VIDT QH 633 .C45 1992 pt.3 Glycolosis 2 (Series Title-Cellular Respiration